Introduction
In industries where precision and reliability are crucial, such as automotive, electronics, and healthcare, quality control in plastic injection molding can make or break a product’s success. Plastic injection molds are used to create thousands of identical parts, each requiring strict adherence to specifications. Without effective quality control processes, businesses risk producing parts with variations, which can lead to functionality issues, costly reworks, and, ultimately, customer dissatisfaction. Implementing quality control in plastic injection molding is not just about detecting defects; it’s about ensuring that every step in the process aligns with quality standards, from mold design and material selection to final inspection.
In this guide, we’ll discuss the importance of quality control in plastic injection molding, best practices for achieving consistent results, and the tools manufacturers can use to uphold high standards. By the end, you’ll have a comprehensive understanding of how quality control contributes to producing safe, reliable plastic parts that meet industry requirements and customer expectations.
Quality control is a critical factor in plastic injection molding, ensuring that every plastic part manufactured meets precise specifications and maintains consistent quality. From automotive to medical applications, plastic parts manufacturers rely on strict quality control practices to prevent defects, minimize waste, and ensure customer satisfaction. In this article, we’ll explore the role of quality control in plastic injection molds, covering best practices, essential tools, and challenges faced by manufacturers in their pursuit of high-quality custom plastic parts.
Section 1: Understanding Quality Control in Plastic Injection Molding
What is Quality Control in Plastic Injection Molding?
Quality control in plastic injection molding is the process of ensuring that every mold and plastic part produced meets specified quality standards. It encompasses every stage of the production process, from initial design and material selection to in-process inspections and final product testing. Quality control is essential in plastic injection molding because even minor variations in mold design or production parameters can result in significant issues, leading to defective or inconsistent products. Effective quality control ensures that each mold functions correctly, producing parts with precise dimensions, surface finishes, and structural integrity.
While quality assurance (QA) refers to the broader process of managing overall production quality, quality control (QC) is more focused on inspection and verification during and after production. In injection molding, QC measures include testing the accuracy of molds, inspecting parts for defects, and validating the consistency of material properties. Through stringent QC practices, manufacturers can ensure that custom plastic parts meet design specifications and perform reliably in their intended applications.
Why Quality Control is Essential in Injection Molding
Quality control is vital in plastic injection molding for multiple reasons, primarily because it affects product consistency, durability, and compliance with industry standards. High-quality molds and consistent parts contribute to a product’s overall functionality and lifespan, reducing the need for costly repairs or replacements. In industries such as healthcare, where safety and performance are paramount, a lack of quality control can lead to severe consequences, including regulatory non-compliance and potential risks to end-users.
Beyond preventing defects, quality control also supports efficiency and cost savings. By identifying issues early in the production process, manufacturers can minimize waste, avoid rework, and streamline their operations. Quality control practices ensure that all parts produced meet the exact same standards, eliminating variations that could affect the assembly and functionality of final products. This consistency not only saves time and resources but also builds a reliable brand reputation, leading to stronger customer loyalty and repeat business.
Key Areas of Quality Control in Injection Molding
Quality control in injection molding spans several critical areas, each of which plays a role in maintaining consistent quality throughout the production cycle. The first area is design and mold validation, which involves ensuring that the mold design is accurate and capable of producing parts that meet specifications. This stage may involve computer-aided design (CAD) validation, prototyping, and pre-production testing.
The next area of quality control is material inspection. Using consistent, high-quality materials is essential, as variations in material properties can lead to issues like warping, brittleness, or poor finish quality. Material inspection includes testing the chemical and physical properties of plastics to confirm that they meet the specified requirements.
Production monitoring is another key area, involving the continuous oversight of production parameters such as temperature, pressure, and cooling time. Consistent monitoring helps prevent deviations that could affect part quality. Finally, final inspection checks each finished part against the design specifications for any visible or structural defects, ensuring that only fully compliant products reach customers.
Section 2: Best Practices in Quality Control for Plastic Injection Molding
Establishing Clear Quality Standards and Specifications
One of the most important steps in quality control for plastic injection molding is establishing clear, measurable quality standards. Before production even begins, manufacturers must define what constitutes an acceptable product. Quality standards outline criteria such as part dimensions, tolerances, surface finish, and material strength, providing a consistent framework that the production team can reference. These standards are often aligned with industry requirements or regulatory guidelines, particularly for fields like automotive or medical devices, where part precision and performance are critical.
A strong quality control process begins with documentation that clearly details these quality specifications. Manufacturers often create detailed drawings and specifications that define all critical dimensions and features of the plastic part. By setting up these standards from the outset, manufacturers can ensure every part meets customer expectations and functions reliably in its intended application. Quality standards act as a benchmark, helping inspectors and production staff quickly identify any deviations or defects and take corrective actions when necessary.
Ensuring Material Quality and Consistency
The materials used in plastic injection molding have a significant impact on the final product’s quality, durability, and performance. Ensuring material quality and consistency is, therefore, a key aspect of quality control. Before production, manufacturers should verify that all raw materials meet the required specifications, such as polymer grade, color, and additives. Consistent material quality is essential for maintaining uniformity across parts and batches, as variations in material properties can lead to issues like uneven shrinkage, warping, or reduced impact resistance.
A best practice for material quality control is batch testing, which involves sampling raw material batches and subjecting them to physical and chemical tests to confirm they meet the necessary standards. Some manufacturers implement advanced techniques like spectrometry or thermal analysis to detect any variations in material composition. By maintaining strict oversight of material quality, manufacturers can reduce the likelihood of producing defective parts and enhance product consistency.
Implementing Mold Design and Validation Processes
The mold itself is central to producing high-quality plastic parts. A well-designed mold ensures that parts are manufactured to exact specifications, while a flawed design can lead to defects such as sink marks, voids, or dimensional inconsistencies. Quality control begins at the design stage, where manufacturers should conduct thorough design reviews, including computer-aided design (CAD) and computer-aided engineering (CAE) analyses. These technologies allow for the simulation of injection molding processes, helping identify potential design flaws before the mold is produced.
Once a mold is created, it undergoes a validation process to confirm that it meets all design and quality requirements. Validation often includes producing prototype parts, testing them under real-world conditions, and refining the mold if needed. This step is particularly crucial for custom plastic parts, where precise dimensions and unique shapes are required. By rigorously validating molds before full production, manufacturers can ensure that each mold functions optimally and yields high-quality, consistent parts.
Process Control and Standardization
In injection molding, controlling the process parameters—such as temperature, pressure, and cooling time—is essential for producing consistent, high-quality parts. Even small variations in these parameters can lead to defects, affecting part quality and performance. Process control involves standardizing these parameters to ensure repeatability, which is crucial for large-scale production runs. Statistical Process Control (SPC) is a commonly used technique that monitors production data and identifies any deviations from established norms.
Standardizing processes ensures that every part produced meets the same quality standards, minimizing the risk of defects and variability. Implementing process control also involves documenting the optimal settings for each part type, which operators can reference to replicate results consistently. By adopting process control practices, plastic injection molding companies can maintain high levels of quality and efficiency across production batches.
Establishing Inspection Protocols at Different Stages
Inspection is a vital component of quality control, and it should be conducted at multiple stages throughout the production process. An effective inspection protocol includes in-process inspections to monitor quality as parts are produced, post-production inspections to identify any defects, and final inspections before parts are shipped to the customer. Each stage serves a different purpose: in-process inspections help catch issues early, post-production inspections assess overall quality, and final inspections ensure customer-ready products.
Establishing standardized checklists for each inspection stage helps streamline the process and ensures that no critical quality criteria are overlooked. These checklists may include specifications for part dimensions, surface finishes, and material properties. By conducting thorough inspections at each stage, manufacturers can catch defects early and reduce the chances of sending flawed products to customers.
Section 3: Quality Control Tools and Equipment in Injection Molding
Common Quality Control Tools for Plastic Injection Molding
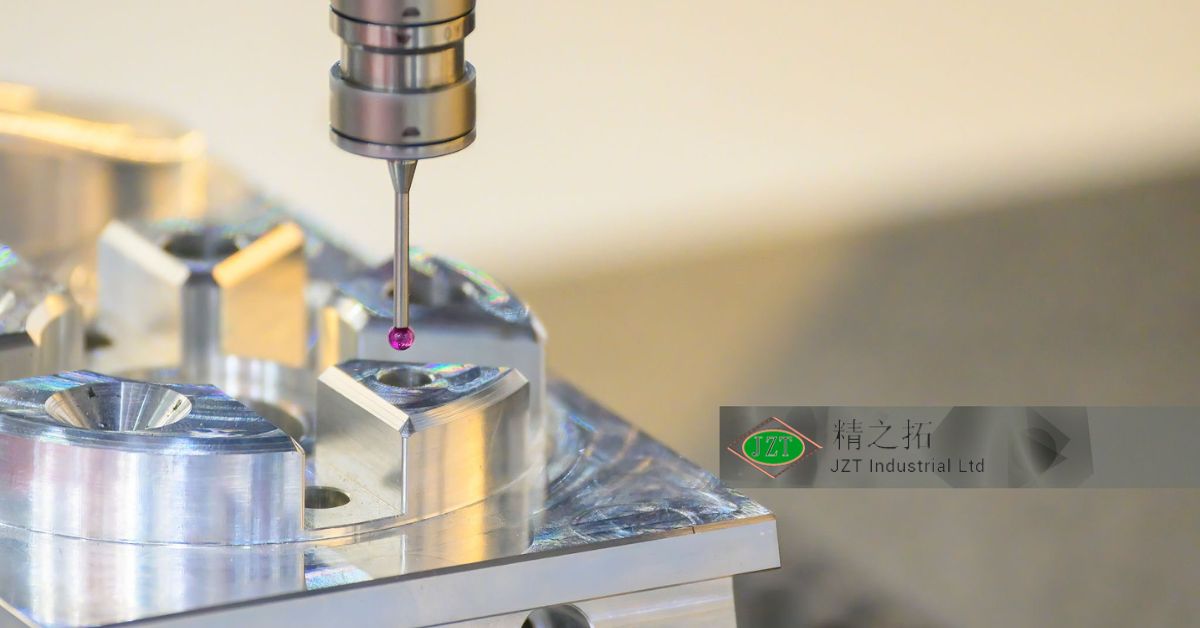
Quality control in plastic injection molding relies on various measurement tools and inspection equipment to verify that parts meet design specifications. Some of the most commonly used tools include Coordinate Measuring Machines (CMMs), which measure precise part dimensions; micrometers, which measure thickness and depth; and optical comparators, which compare part features against the original design. These tools allow inspectors to assess parts with a high degree of accuracy, ensuring that they meet all critical dimensions.
Each of these tools serves a specific purpose in the inspection process. For example, CMMs are ideal for measuring complex geometries, while micrometers are best suited for thickness measurements. Optical comparators, on the other hand, are valuable for visual inspections, allowing inspectors to compare the part’s profile to a reference. By using these tools effectively, manufacturers can maintain tight tolerances and deliver consistent results in their plastic injection molding operations.
Advanced Quality Control Technologies
In addition to basic inspection tools, many manufacturers use advanced technologies to enhance their quality control efforts. CT scanners and 3D scanners offer non-destructive testing capabilities, allowing manufacturers to examine internal structures and verify the accuracy of complex geometries without damaging the part. These technologies are particularly useful for parts with intricate designs or internal cavities, where traditional measurement tools may be insufficient.
Digital microscopes are another advanced tool used in quality control, providing high-resolution images of part surfaces to detect flaws such as scratches, burrs, or microscopic cracks. By investing in advanced quality control technologies, manufacturers can achieve higher precision, reduce the risk of defects, and improve overall product quality, particularly for custom plastic parts that require complex designs and exact specifications.
In-Process Monitoring and Data Collection Tools
Real-time monitoring tools are essential for maintaining quality throughout the injection molding process. In-process monitoring tools, such as thermocouples (which measure temperature), pressure sensors, and flow meters, track critical parameters and ensure they remain within acceptable ranges. Data collected from these tools is analyzed to detect any deviations that could lead to defects, allowing operators to make adjustments on the fly and prevent quality issues from developing.
Data collection systems play a significant role in quality control by compiling real-time data on production parameters. This data enables manufacturers to conduct trend analysis, identifying any recurring issues that may require adjustments in mold design, material selection, or process settings. By implementing in-process monitoring and data collection tools, manufacturers can achieve greater consistency, reduce waste, and optimize their injection molding operations for better results.
Automation and AI in Quality Control
Automation and artificial intelligence (AI) are transforming quality control in plastic injection molding, offering new ways to enhance accuracy and efficiency. Machine vision systems, for example, use cameras and AI algorithms to inspect parts in real-time, identifying defects that may be difficult for the human eye to detect. These systems can operate continuously, enabling manufacturers to inspect large volumes of parts without the risk of human error.
AI-powered analytics can also assist with quality control by analyzing production data and identifying patterns that may indicate quality issues. For example, AI can detect trends in temperature fluctuations or material inconsistencies that could lead to defects, allowing manufacturers to address problems proactively. By integrating automation and AI into quality control processes, plastic parts manufacturers can achieve higher precision, reduce costs, and improve product quality across their operations.
Section 4: Common Quality Control Challenges in Injection Molding
Variability in Material Properties
Material variability is a common challenge in plastic injection molding quality control, as even minor differences in material composition or properties can affect part performance. Variations in resin batches, additives, or colorants can lead to inconsistencies such as warping, shrinkage, or reduced impact strength. To mitigate these risks, manufacturers often implement strict material testing procedures, such as thermal analysis and tensile testing, to verify material properties before use.
Standardizing material suppliers and conducting batch tests can also reduce variability, ensuring that materials meet the required specifications. By maintaining tight control over material quality, manufacturers can minimize the risk of defects and produce parts with consistent properties.
Mold Wear and Maintenance Issues
Molds are subject to wear over time, which can lead to quality issues if not properly managed. Mold wear can cause defects such as flash, sink marks, or poor surface finishes, as worn molds may no longer produce parts with the desired accuracy. Proactive mold maintenance is essential to minimize these issues. Manufacturers should conduct regular inspections and cleaning to remove residues, inspect for signs of wear, and replace worn components.
In addition, monitoring mold performance data can help detect early signs of wear, allowing for timely maintenance and preventing costly downtime. By implementing a preventive maintenance schedule, manufacturers can extend mold life, reduce quality issues, and maintain production efficiency.
Inconsistencies in Process Parameters
Variations in process parameters, such as temperature, pressure, and cooling time, can lead to defects in plastic injection molded parts. For example, an incorrect cooling time can result in warping or uneven shrinkage, while variations in pressure can cause voids or sink marks. Maintaining consistent process parameters is crucial for producing high-quality parts, especially in high-volume production.
Process control methods, such as Statistical Process Control (SPC), enable manufacturers to monitor these parameters and detect any deviations in real-time. By standardizing processes and using data-driven controls, manufacturers can ensure that every part produced meets quality specifications, reducing the risk of defects and improving product consistency.
Human Error in Quality Control Processes
Human error is a common challenge in quality control, as manual inspections and measurements are susceptible to inaccuracies. Mistakes in measurement, inspection, or process adjustments can lead to defects, affecting part quality and overall productivity. To reduce human error, manufacturers can implement automation, provide training for quality control staff, and use standardized inspection protocols.
Automation tools, such as machine vision systems, can handle repetitive inspection tasks with higher accuracy than human inspectors. Regular training programs also help staff stay updated on quality control practices and reduce errors in manual processes. By addressing human error through training and automation, manufacturers can enhance the reliability and accuracy of their quality control processes.
Section 5: Quality Control Standards and Certifications
Overview of Key Quality Standards in Injection Molding
Quality standards provide a framework for plastic injection mold manufacturers, ensuring that every part produced meets industry and regulatory requirements. Common standards include ISO 9001, which sets guidelines for quality management systems across industries, and ISO 13485, which specifically focuses on quality management for medical devices. These certifications verify that a plastic parts manufacturer adheres to strict quality control processes, providing reassurance to customers that the supplier is committed to high standards.
For industries like automotive, IATF 16949 is particularly relevant, setting standards that address specific requirements for quality, traceability, and risk management in automotive parts production. These certifications not only reinforce quality control practices but also establish a standardized approach that reduces the risk of errors and defects. Manufacturers holding these certifications must undergo regular audits, demonstrating their commitment to continuous improvement and adherence to quality protocols.
Industry-Specific Standards and Compliance Requirements
Different industries have unique quality control standards and compliance requirements, tailored to the needs of their specific applications. For example, medical device manufacturers must comply with FDA regulations and standards like ISO 13485 to ensure that all products are safe for patient use. This includes rigorous testing, documentation, and traceability requirements that verify each part’s safety and reliability. In the automotive industry, adherence to IATF 16949 is essential, as it covers requirements like defect prevention and continuous improvement, both critical for parts that will endure high stress and varying conditions.
Compliance with these standards not only protects businesses from regulatory penalties but also boosts confidence in their products. By partnering with certified suppliers, businesses can mitigate risks, ensure regulatory compliance, and assure customers that their products are manufactured to the highest standards. Compliance is particularly crucial for custom plastic parts used in regulated industries, where failure to meet industry-specific standards could lead to severe consequences.
Importance of Certification and Audits
Certifications like ISO 9001, ISO 13485, and IATF 16949 serve as a testament to a manufacturer’s commitment to quality. These certifications require companies to establish and maintain quality control processes that consistently produce defect-free products. Achieving certification often involves an in-depth review of manufacturing practices, quality control systems, and staff training programs, ensuring that every aspect of production meets stringent quality requirements.
Regular audits, both internal and external, are also a cornerstone of certified quality management systems. Internal audits allow companies to identify potential issues and implement corrective actions before they impact product quality, while external audits provide an objective assessment from accredited certification bodies. By consistently meeting certification and audit requirements, plastic parts manufacturers demonstrate their dedication to quality, reliability, and compliance, which reassures customers and enhances brand reputation.
Section 6: Steps for Implementing a Quality Control System in Injection Molding
Defining Quality Objectives and Key Performance Indicators (KPIs)
The first step in building a robust quality control system is defining clear quality objectives and key performance indicators (KPIs). These objectives outline the standards a manufacturer aims to achieve, such as defect-free production, minimal waste, or consistent part dimensions. KPIs provide measurable benchmarks for these objectives, allowing companies to monitor performance over time. Common KPIs in injection molding quality control include defect rates, inspection pass rates, and production efficiency metrics.
Setting specific, measurable quality objectives helps align the entire production team around a shared goal. These objectives should reflect both industry standards and customer expectations, creating a quality-focused culture within the organization. By regularly reviewing KPIs, manufacturers can track progress, identify areas for improvement, and continuously refine their quality control processes.
Developing a Quality Control Plan
A quality control plan is a structured document that details each step in the quality control process, including inspection points, testing methods, and acceptable quality thresholds. The plan should cover every stage of production, from raw material inspection to final product verification, ensuring that all critical aspects of quality are addressed. Additionally, it should outline protocols for handling defective parts, such as rework procedures or corrective actions.
A well-designed quality control plan serves as a guide for operators, inspectors, and supervisors, standardizing quality checks and minimizing variability across production runs. By implementing a thorough quality control plan, manufacturers can ensure consistency, reduce waste, and enhance customer satisfaction by delivering reliable, high-quality custom plastic parts.
Training Staff on Quality Control Practices
Quality control relies not only on tools and protocols but also on skilled personnel who understand the importance of maintaining high standards. Training programs should educate staff on inspection techniques, equipment usage, and the importance of adhering to quality control standards. Training should also cover specific quality criteria relevant to each production stage, from mold setup and material handling to in-process inspections and final checks.
Continuous training ensures that staff are equipped to identify defects, troubleshoot issues, and uphold quality standards in their daily work. Regularly scheduled refresher courses and updates on industry standards can further enhance quality control, enabling teams to stay current with evolving best practices and technologies in plastic injection molding.
Implementing Data Collection and Analysis Systems
Data collection is a cornerstone of quality control, enabling manufacturers to monitor key variables and make data-driven decisions. By implementing data collection systems, companies can track production parameters, detect trends, and identify recurring issues that may require adjustments. For instance, temperature and pressure data can be used to identify variations that affect part quality, while inspection data can reveal common defect patterns.
Analysis tools such as Statistical Process Control (SPC) can further enhance quality control by monitoring production data in real-time, alerting operators to any deviations. Regular data analysis allows manufacturers to pinpoint the root causes of quality issues, facilitating continuous improvement. With data-driven insights, plastic parts manufacturers can optimize their processes, reduce variability, and maintain high quality across production runs.
Conducting Regular Reviews and Continuous Improvement
Quality control is not a one-time effort but an ongoing process of refinement and improvement. Conducting regular reviews of quality data, inspection reports, and customer feedback helps manufacturers identify areas for improvement and implement corrective actions. Continuous improvement methodologies, such as Six Sigma or Lean Manufacturing, provide frameworks for systematically enhancing quality and reducing waste.
Through continuous improvement, manufacturers can address recurring issues, adapt to changing customer needs, and maintain competitive advantages in quality and efficiency. Regular reviews and updates to the quality control plan ensure that processes remain aligned with industry standards and that manufacturers continue to deliver high-quality plastic injection molded parts.
Section 7: Case Studies and Real-World Examples
Case Study 1: Successful Implementation of Quality Control in Injection Molding
A well-known automotive manufacturer partnered with a plastic injection mold supplier who implemented a rigorous quality control system focused on precision and defect reduction. By establishing stringent inspection protocols, conducting material consistency tests, and utilizing real-time data monitoring, the supplier reduced defects by 25% and significantly improved part durability. This case demonstrates how adopting quality control best practices can improve product reliability and reduce production costs.
Case Study 2: Reducing Defects Through Advanced Quality Control Tools
A medical device company required high-precision plastic parts for their surgical equipment. By working with a supplier who utilized advanced 3D scanners and automated inspection tools, the company reduced defect rates and achieved high compliance with regulatory standards. The supplier’s investment in quality control tools allowed for detailed inspections of complex parts, ensuring that each met the strict dimensional and safety standards required for medical devices.
Case Study 3: Quality Control in High-Volume Production
In the consumer electronics sector, a manufacturer faced challenges in maintaining quality across high-volume orders. By implementing Statistical Process Control (SPC) and real-time monitoring, the supplier managed to maintain consistent quality, even as production volumes increased. This approach minimized variability, ensuring that every part met quality standards and reducing the need for costly rework.
Lessons Learned from Quality Control Failures
In a notable quality control failure, a plastic parts manufacturer produced a batch of automotive components with dimensional discrepancies due to inadequate mold maintenance. These defects led to costly recalls and significant damage to the manufacturer’s reputation. The lesson underscores the importance of preventive maintenance, rigorous inspection protocols, and adherence to quality control best practices.
Frequently Asked Questions (FAQs)
- What are the main benefits of quality control in plastic injection molding?\
Quality control ensures consistent product quality, reduces defect rates, minimizes waste, and enhances customer satisfaction, making it essential for reliable plastic parts manufacturing. - What tools are commonly used for quality control in injection molding?\
Common tools include Coordinate Measuring Machines (CMMs), optical comparators, micrometers, and advanced technologies like CT scanners and 3D scanners for precise measurements. - How can quality control reduce defects in plastic parts production?\
Quality control helps identify defects early, allowing manufacturers to make adjustments in real-time, maintain consistent process parameters, and ensure all parts meet specifications. - What role does data collection play in quality control?\
Data collection enables manufacturers to track production variables, conduct trend analysis, and identify root causes of quality issues, which supports continuous improvement and defect reduction. - Why are certifications like ISO 9001 important for injection molding quality?\
Certifications like ISO 9001 provide a standardized framework for quality management, ensuring that manufacturers follow best practices and consistently meet industry standards.
Conclusion
Quality control is the backbone of reliable plastic injection molding, ensuring that every part produced meets high standards of consistency, durability, and precision. From setting quality objectives and establishing inspection protocols to utilizing advanced tools and conducting regular audits, effective quality control practices support manufacturing excellence. By investing in quality control, manufacturers can reduce defects, improve efficiency, and deliver safe, high-quality custom plastic parts that meet both customer and regulatory expectations. For any business involved in injection molding, prioritizing quality control is essential not only for product success but for building a reputation as a trusted and dependable manufacturer in a competitive industry.