Introduction
In the healthcare industry, product safety and reliability are paramount. Medical-grade plastic injection molds play a critical role in ensuring that plastic components used in medical devices, instruments, and healthcare products meet the highest standards of quality, precision, and safety. These custom plastic parts, made by specialized plastic parts manufacturers, serve as the backbone of various healthcare applications, where even minor defects or contamination can lead to serious consequences for patient health.
Medical-grade plastic injection molding goes beyond traditional molding processes by enforcing stricter guidelines on materials, precision, and contamination control, essential for safeguarding patient outcomes. In this article, we’ll dive into the importance of these molds in healthcare, explore the detailed requirements they meet, and examine why they are indispensable for the production of safe and reliable medical products.
Section 1: Understanding Medical-Grade Plastic Injection Molds
What Are Medical-Grade Plastic Injection Molds?
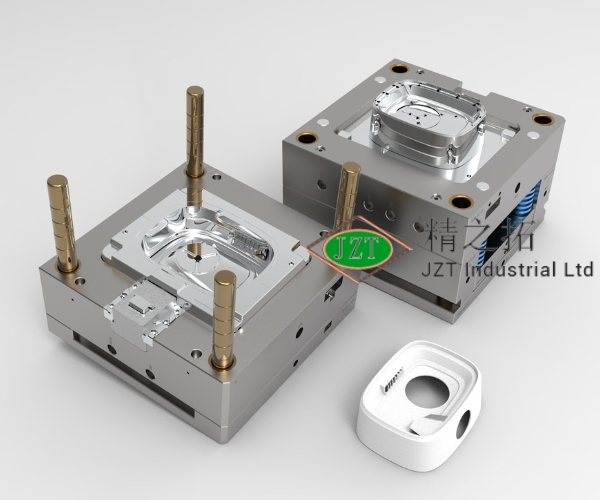
Medical-grade plastic injection molds are specialized tools used in the manufacturing of plastic parts intended for medical applications. Unlike standard molds, these molds meet stringent requirements set forth by healthcare regulations, focusing on hygiene, precision, and durability. By creating high-quality components for medical devices, plastic injection molds ensure that each part is produced accurately, with zero tolerance for defects or contamination that could jeopardize patient safety.
In practice, a plastic injection mold suitable for medical use is manufactured with extreme precision, often utilizing advanced technology to achieve tolerances within micrometers. This level of precision is vital, as medical devices frequently involve intricate designs with delicate structures that require exact replication across millions of parts. From syringes to surgical instruments, these parts are not only complex but must be consistent, clean, and sterile to be safe for human use.
The Role of Medical-Grade Molds in Healthcare
In the healthcare industry, medical-grade molds are foundational to the safe production of a wide range of devices. Their applications include life-saving devices such as pacemakers, syringes, catheters, and surgical instruments. Medical-grade plastic parts manufactured through injection molding must meet precise specifications because a minor flaw in a product like an implantable device could lead to malfunction, causing harm to patients or even proving fatal.
Medical-grade plastic injection molds ensure each part’s integrity and durability. Given the sterile environment in which these products are used, the manufacturing process must prevent contamination. This is why molds for medical parts are produced in cleanrooms—controlled environments free from dust and bacteria. Moreover, medical-grade molds allow healthcare providers to have consistent, reliable products that meet the high standards required in life-critical environments.
Material Selection for Medical-Grade Molds
One of the key aspects of medical-grade plastic injection molding is the careful selection of materials. Unlike standard plastics used in consumer products, medical-grade plastics are rigorously tested to ensure biocompatibility, non-toxicity, and resilience under various sterilization methods. Common materials include polypropylene, polycarbonate, polyetheretherketone (PEEK), and thermoplastic elastomers, which meet strict criteria for durability and patient safety.
Biocompatibility is essential for any material used in medical products that come into direct contact with human tissue or bodily fluids. These materials must be non-reactive to avoid adverse effects like allergies or toxicity. Additionally, they must withstand sterilization processes—often involving high temperatures, radiation, or chemical treatments—without degrading or releasing harmful substances. Material choice in medical-grade molds, therefore, directly influences the overall safety and performance of the final product, ensuring that it remains durable and safe under various medical conditions.
Section 2: Why Quality Control is Critical in Medical Molding
Importance of Stringent Quality Standards
In the production of medical-grade plastic parts, quality control is not just a requirement; it is a critical pillar for safeguarding patient safety. Quality control measures are rigorous, aimed at eliminating potential defects and ensuring that every single part produced meets exact specifications. Unlike other industries, where minor imperfections might be acceptable, healthcare demands near-perfect consistency, as even the smallest defect can pose a risk to patient health.
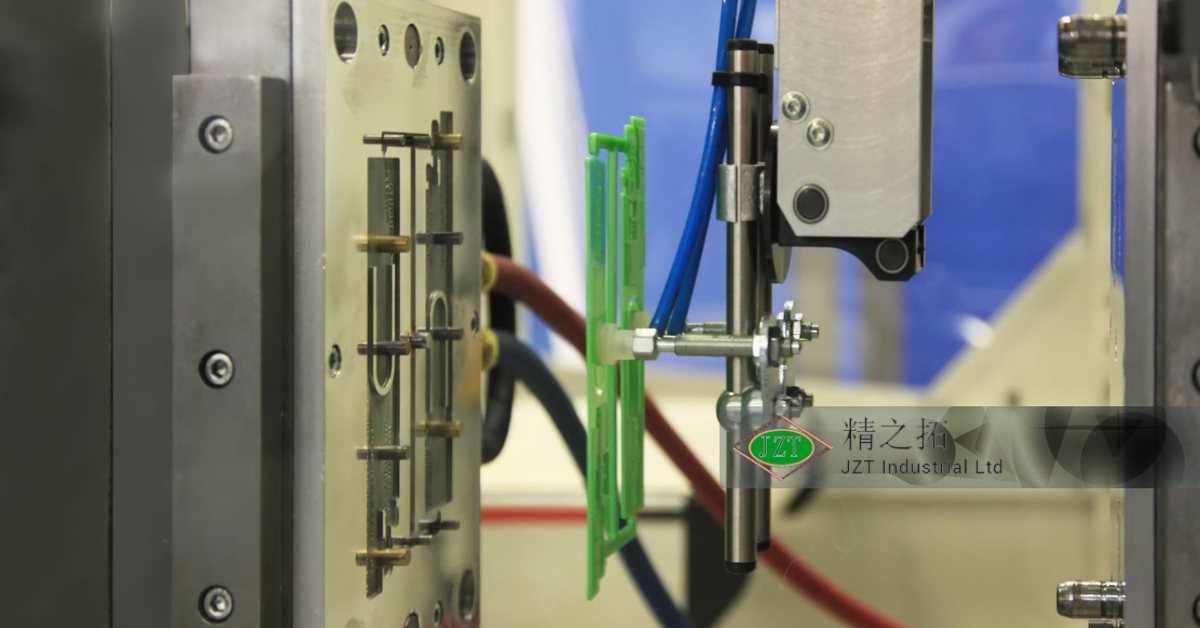
Each stage of production is closely monitored, from the initial mold design to the final inspection. For example, medical-grade plastic injection molds are subject to frequent testing and evaluation to ensure that they produce parts with exact dimensions and no irregularities. Contamination control is another major aspect of quality in medical molding. To maintain a sterile environment, many manufacturers employ cleanroom facilities, which prevent dust, bacteria, and other contaminants from coming into contact with molds and materials. This is especially crucial in manufacturing parts that will be used in or near the human body, where contamination can lead to infection or other health risks.
Precision in Mold Design and Manufacturing
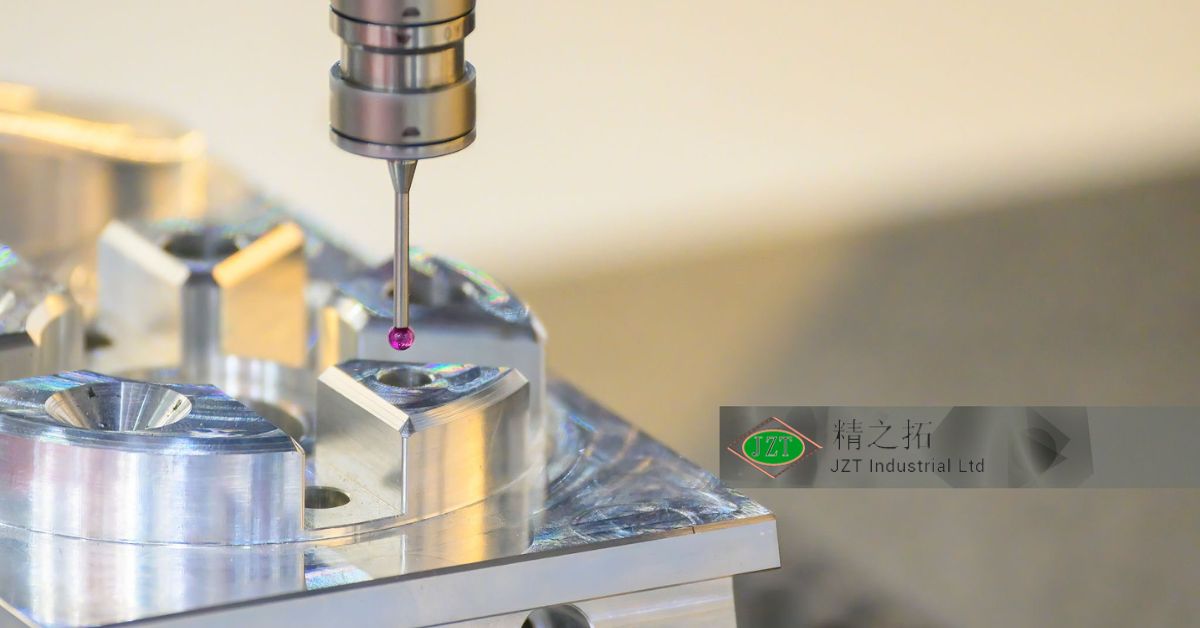
The design and manufacturing of molds for medical-grade applications require extreme precision. Unlike standard molds, which may allow for minor variations, medical-grade molds are crafted with tolerances as tight as a few micrometers. Achieving this level of accuracy involves using advanced equipment, such as computer numerical control (CNC) machines and coordinate measuring machines (CMM), to produce highly detailed molds that can create components with exact specifications.
Precision is essential because medical devices often involve intricate and delicate parts that must function seamlessly within the human body or in contact with delicate tissue. A mold with even a small defect could result in parts that do not fit correctly, break easily, or fail to function as intended. For example, a poorly manufactured catheter could have sharp edges or weak spots, leading to injury during insertion. With precise mold design and manufacturing processes, plastic parts manufacturers can ensure that each piece produced is identical to the last, thus maintaining consistent quality and safety across all products.
Injection Molding Process Control
The process of injection molding involves several critical stages: clamping, injection, cooling, and ejection. In medical-grade molding, each of these stages is tightly controlled to maintain the high-quality standards required for healthcare applications. During the clamping phase, the mold is secured to withstand high pressures. In the injection phase, melted plastic is injected into the mold cavity, filling every intricate detail of the design. Cooling solidifies the plastic, while the ejection phase removes the molded part without damaging its delicate structure.
Process control in each phase is vital because any deviation can compromise the final product’s integrity. For instance, if the cooling process is too rapid, it can create stresses within the plastic that weaken the part. Similarly, incorrect injection pressure can result in incomplete filling, leaving voids or weak spots in the part. By carefully controlling each step, plastic parts manufacturers can ensure that every component meets the stringent safety and performance standards required for medical use.
Section 3: Regulatory Standards and Compliance for Medical-Grade Molds
Key Regulatory Bodies and Standards
Medical-grade plastic injection molds must adhere to strict regulations imposed by bodies like the U.S. Food and Drug Administration (FDA) and the International Organization for Standardization (ISO). Key standards include ISO 13485, which outlines quality management systems specific to medical devices, and FDA guidelines that regulate the safety and effectiveness of medical devices. These regulatory standards serve as a framework for maintaining the quality and safety of medical devices, ensuring that products meet uniform standards across manufacturers.
Compliance with these regulations involves not only adhering to manufacturing best practices but also documenting each step to provide a clear trace of the product’s journey from design to final product. Manufacturers are often required to submit their quality control procedures, testing results, and compliance records for inspection. This level of oversight is designed to protect patient health and maintain a high level of trust in healthcare products by ensuring they are safe, reliable, and effective.
Why Compliance Matters for Healthcare Product Safety
Compliance with regulatory standards is critical for ensuring that medical-grade plastic parts are safe for use in healthcare settings. When manufacturers adhere to guidelines like ISO 13485, they commit to practices that minimize risks, such as contamination, defects, and improper design. The impact of non-compliance can be severe, leading to product recalls, legal consequences, and most importantly, patient harm. A defective part used in a medical device could fail during operation, endangering the patient and eroding trust in healthcare providers and manufacturers alike.
Regulatory compliance in medical-grade injection molding includes regular audits, testing, and validation processes. Audits ensure that manufacturers continually meet regulatory standards, while testing and validation confirm that products meet the necessary performance criteria. Compliance also builds confidence among healthcare providers, who rely on the consistent quality of medical devices to provide safe care for patients.
Documentation and Traceability in Medical Mold Production
An essential part of regulatory compliance is the documentation and traceability of every step in the manufacturing process. Medical device manufacturers are required to maintain meticulous records, detailing each aspect of the production, testing, and inspection processes. This documentation is not only crucial for regulatory audits but also for internal quality control, enabling manufacturers to trace any issue back to its source.
Traceability is particularly important in the event of a product recall. By maintaining detailed records, manufacturers can quickly identify and isolate defective batches, reducing the risk of widespread issues and ensuring that corrective actions can be taken promptly. For healthcare providers and patients, this traceability translates to a higher level of trust in the safety and reliability of medical-grade plastic parts.
Section 4: The Impact of Medical-Grade Molding on Patient Safety
Reducing the Risk of Contamination
One of the main benefits of using medical-grade molds in healthcare is the significant reduction in contamination risk. Contamination poses a serious threat in medical settings, where even trace amounts of foreign material can lead to infections and complications. Medical-grade plastic injection molding is conducted in cleanrooms, which are specially designed environments that control factors like dust, bacteria, and airborne particles.
In a cleanroom setting, medical-grade molds are manufactured and handled with strict protocols to prevent contamination at every stage of production. From using sterilized equipment to employing specialized filtration systems, cleanroom environments significantly minimize the risk of contaminants reaching the final product. For medical devices that come into direct contact with a patient’s body, such as catheters or implants, this level of control is essential to ensure patient safety and prevent healthcare-associated infections.
Achieving Consistency and Accuracy in Medical Components
Consistency is paramount when producing medical devices and components. Medical-grade plastic parts must be produced with exacting precision to ensure that they function as intended across all applications. For instance, an inconsistency in the size of a molded part could lead to leaks in devices that deliver medication or could compromise the structural integrity of surgical instruments.
With advanced medical-grade plastic injection molding, manufacturers can produce thousands of identical parts with minimal variation. This is achieved through rigorous quality control and precision manufacturing processes, ensuring that every part meets the exact specifications required. For healthcare providers, consistent quality means they can rely on their tools and devices to perform reliably, contributing to better patient outcomes and safer healthcare practices.
Durability and Longevity of Medical-Grade Molded Products
Medical devices often undergo repeated use, and many are subjected to harsh cleaning and sterilization processes that can degrade standard plastics. Medical-grade plastic injection molding uses high-quality materials that are selected for their durability, resilience, and resistance to chemicals. This ensures that the molded parts remain functional and safe even after multiple sterilization cycles or exposure to aggressive cleaning agents.
Durability is essential, especially in critical medical applications where device failure is not an option. For example, implants must last for years within the human body without degrading, while surgical tools need to withstand repeated use without losing their functionality. By using durable materials and strict manufacturing standards, medical-grade plastic injection molding delivers products that offer long-lasting performance, reducing the need for frequent replacements and contributing to overall healthcare cost savings.
Section 5: Advanced Technologies in Medical Injection Molding
Technological Innovations in Medical Molding
Medical-grade plastic injection molding has seen significant advancements in technology, with innovations such as computer-aided design (CAD) and computer-aided manufacturing (CAM) enhancing the precision and reliability of mold production. These tools allow manufacturers to design intricate molds with high accuracy, enabling the production of complex parts that meet the stringent requirements of healthcare applications. By simulating the molding process digitally, CAD and CAM reduce errors, optimize design, and ensure that every detail is accounted for before production begins.
These technologies also enable faster prototyping, allowing manufacturers to test and refine designs before full-scale production. Rapid prototyping is especially valuable in the medical industry, where timely access to reliable devices can impact patient care. For manufacturers, using these advanced technologies translates to improved efficiency, reduced waste, and higher-quality end products that meet the exact needs of healthcare providers.
Automated Quality Control Systems
Automation has revolutionized quality control in medical-grade molding, minimizing human error and enhancing precision. Automated quality control systems, such as vision inspection technology and real-time monitoring, allow for continuous oversight of the production process. By using sensors and cameras, these systems detect even the smallest deviations, alerting operators to potential issues before they affect the entire production batch.
Automated quality control not only improves the accuracy of medical-grade plastic parts but also increases production efficiency. By quickly identifying and correcting defects, these systems reduce downtime and minimize waste. In an industry where consistency is crucial, automation provides a reliable solution for maintaining high-quality standards across large production volumes, ensuring that every part meets the requirements for safe medical use.
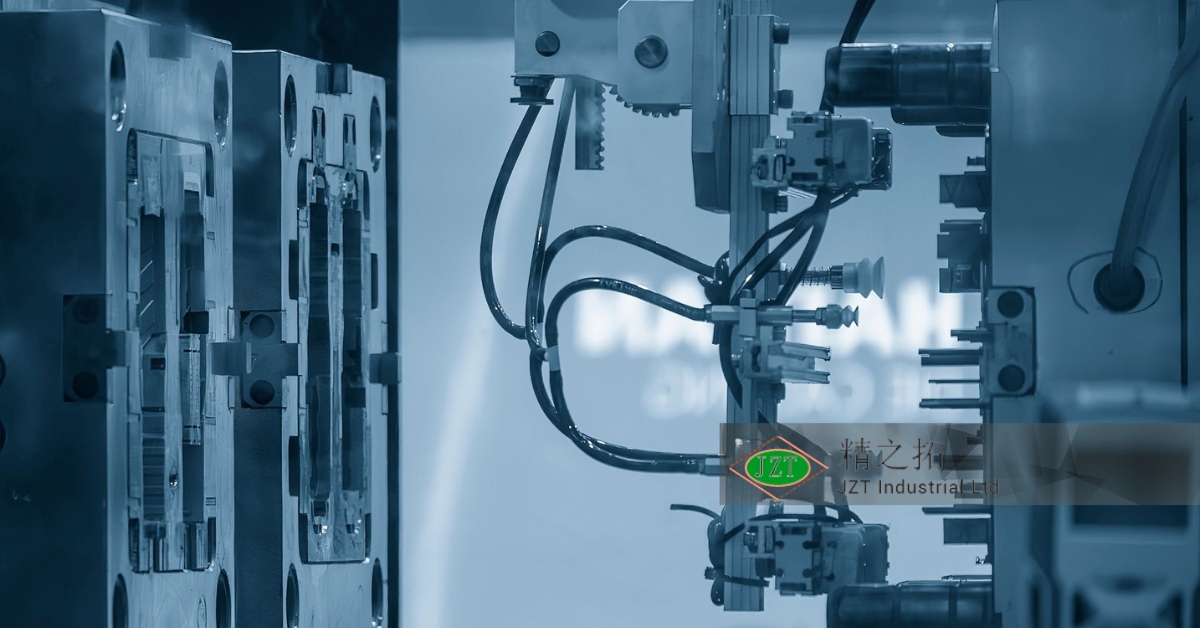
The Role of Robotics in Medical Molding Production
Robotics plays a significant role in modern medical-grade plastic injection molding, especially in tasks that require high precision and cleanliness. Robots can handle materials and components without human intervention, reducing the risk of contamination and improving consistency. In processes that require repetitive actions, such as part inspection or packaging, robots provide reliable and consistent performance, enhancing efficiency and precision.
The use of robotics in medical molding is particularly valuable in cleanroom environments, where reducing human presence minimizes the risk of contamination. Robots can perform tasks like placing and removing parts from molds, reducing manual handling and maintaining the sterile environment required for medical-grade manufacturing. For healthcare providers and patients, robotics in production translates to safer, cleaner, and more reliable medical products.
Section 6: Key Considerations in Choosing a Medical-Grade Molding Supplier
Experience and Expertise in Medical-Grade Standards
Selecting the right plastic parts manufacturer for medical-grade molding is crucial for ensuring product quality and regulatory compliance. Manufacturers with experience in medical-grade standards understand the unique requirements of the healthcare industry, including precision, sterility, and regulatory adherence. Partnering with an experienced manufacturer provides peace of mind, as they are more likely to have the necessary certifications, quality control processes, and cleanroom facilities to produce safe medical-grade plastic parts.
For healthcare providers, working with an experienced supplier means they can trust the quality and reliability of the products. Experienced suppliers also have a deeper understanding of the materials, processes, and regulatory requirements specific to medical-grade molding, ensuring that every aspect of production aligns with industry standards and meets the expectations of healthcare providers.
Inspection and Quality Assurance Capabilities
A supplier’s quality assurance capabilities are essential for producing high-quality medical-grade parts. Before selecting a manufacturer, healthcare companies should inquire about their inspection methods, quality control equipment, and documentation processes. Manufacturers that employ advanced inspection systems, such as CMM testing and vision inspection, offer higher accuracy and consistency, reducing the risk of defects and ensuring that every product meets the necessary standards.
Quality assurance also extends to documentation, which is vital for regulatory compliance. Reliable suppliers maintain detailed records of each production step, including materials used, inspection results, and quality control protocols. This traceability is valuable for regulatory audits and allows for rapid identification and resolution of issues, ensuring continuous compliance and patient safety.
Production Capacity and Timeliness
Production capacity is another crucial factor when selecting a plastic parts manufacturer for medical-grade applications. Healthcare providers often require large volumes of parts, sometimes on short notice, making it essential to work with suppliers who can meet high production demands. Additionally, timely production is critical in healthcare, where delays in the supply chain can impact patient care.
A manufacturer with sufficient capacity and efficient resource allocation can ensure that products are delivered on time, even for large-scale orders. By partnering with a supplier who can meet both quality and quantity requirements, healthcare providers can maintain reliable access to the medical-grade parts they need, avoiding disruptions in care and treatment.
Section 7: Real-World Applications and Case Studies
Case Study Examples of Successful Medical-Grade Injection Molding Projects
Real-world examples help illustrate the impact of medical-grade plastic injection molding on patient safety and healthcare quality. For instance, consider a case where a manufacturer produced plastic components for ventilators during a pandemic. By adhering to medical-grade standards, they ensured that each part met stringent safety requirements, enabling healthcare providers to deliver life-saving care without concerns over product reliability.
Case studies like these demonstrate how medical-grade molding contributes to healthcare’s resilience, particularly during emergencies. The lessons from these projects highlight the importance of regulatory compliance, quality control, and responsive production in meeting the unique demands of healthcare applications.
Comparing Results: Medical-Grade vs. Non-Medical Grade Molds
Medical-grade and non-medical grade molds differ significantly in their performance, especially in terms of safety, reliability, and durability. Non-medical grade molds may produce parts with minor defects or variations that, while acceptable in other industries, are unsuitable for healthcare. By contrast, medical-grade molds ensure higher consistency and cleanliness, reducing risks for patients.
For instance, medical-grade molds undergo additional sterilization and contamination control measures, preventing foreign particles from contaminating the parts. This level of quality is critical in healthcare, where even minor impurities can compromise patient safety. Comparing the results of medical-grade versus standard molds reveals the indispensable role of strict quality control and regulatory adherence in ensuring reliable healthcare products.
Section 8: Future Trends in Medical-Grade Plastic Injection Molding
Innovations in Biocompatible Materials
As technology advances, biocompatible materials continue to evolve, providing safer and more effective options for medical-grade plastic injection molding. Researchers are developing materials that degrade safely within the body or exhibit improved durability under sterilization. These innovations expand the possibilities for implantable devices and disposable medical products, making healthcare more accessible and safer for patients worldwide.
The Rise of Smart Injection Molding Technologies
Smart manufacturing tools, such as IoT-enabled molds and data-driven quality control, are transforming medical-grade injection molding. By monitoring the injection process in real-time, manufacturers can quickly identify and address issues, improving consistency and reducing waste. These technologies provide deeper insights into production, allowing manufacturers to make data-informed adjustments that optimize both quality and efficiency.
Sustainability and Environmental Responsibility in Medical Molding
Sustainability is increasingly important in medical manufacturing. With a focus on reducing waste, medical-grade plastic injection molding is adopting eco-friendly practices. Manufacturers are exploring biodegradable plastics and reducing the carbon footprint of their production processes, contributing to a more sustainable healthcare industry that aligns with environmental responsibility.
Frequently Asked Questions (FAQs)
- What makes a mold “medical-grade”? Medical-grade molds meet strict standards for precision, sterility, and material safety, designed specifically for healthcare applications where patient safety is paramount.
- Are there specific certifications for medical injection molding? Yes, certifications like ISO 13485 ensure that manufacturers adhere to quality management standards specific to medical devices.
- How does medical-grade molding prevent contamination? Medical-grade molding is often conducted in cleanrooms, where environmental controls prevent dust and bacteria from contaminating the parts.
- What are the risks of using non-medical-grade molds for healthcare products? Non-medical-grade molds may produce parts with contaminants, defects, or material inconsistencies, which can compromise patient safety.
- How does the cost of medical-grade molding compare to standard molding? Medical-grade molding tends to be more expensive due to strict quality controls, advanced materials, and regulatory compliance, but the added safety is essential in healthcare.
Conclusion
Medical-grade plastic injection molds play an irreplaceable role in healthcare, delivering safe, precise, and reliable plastic parts for medical devices. Through rigorous quality control, regulatory compliance, and advanced technology, these molds ensure that healthcare products meet the highest standards of safety and efficacy. For healthcare providers, choosing a reliable plastic parts manufacturer with expertise in medical-grade molding is essential for delivering safe patient care. By understanding and prioritizing medical-grade molds, the healthcare industry continues to provide safe, innovative, and life-saving products.